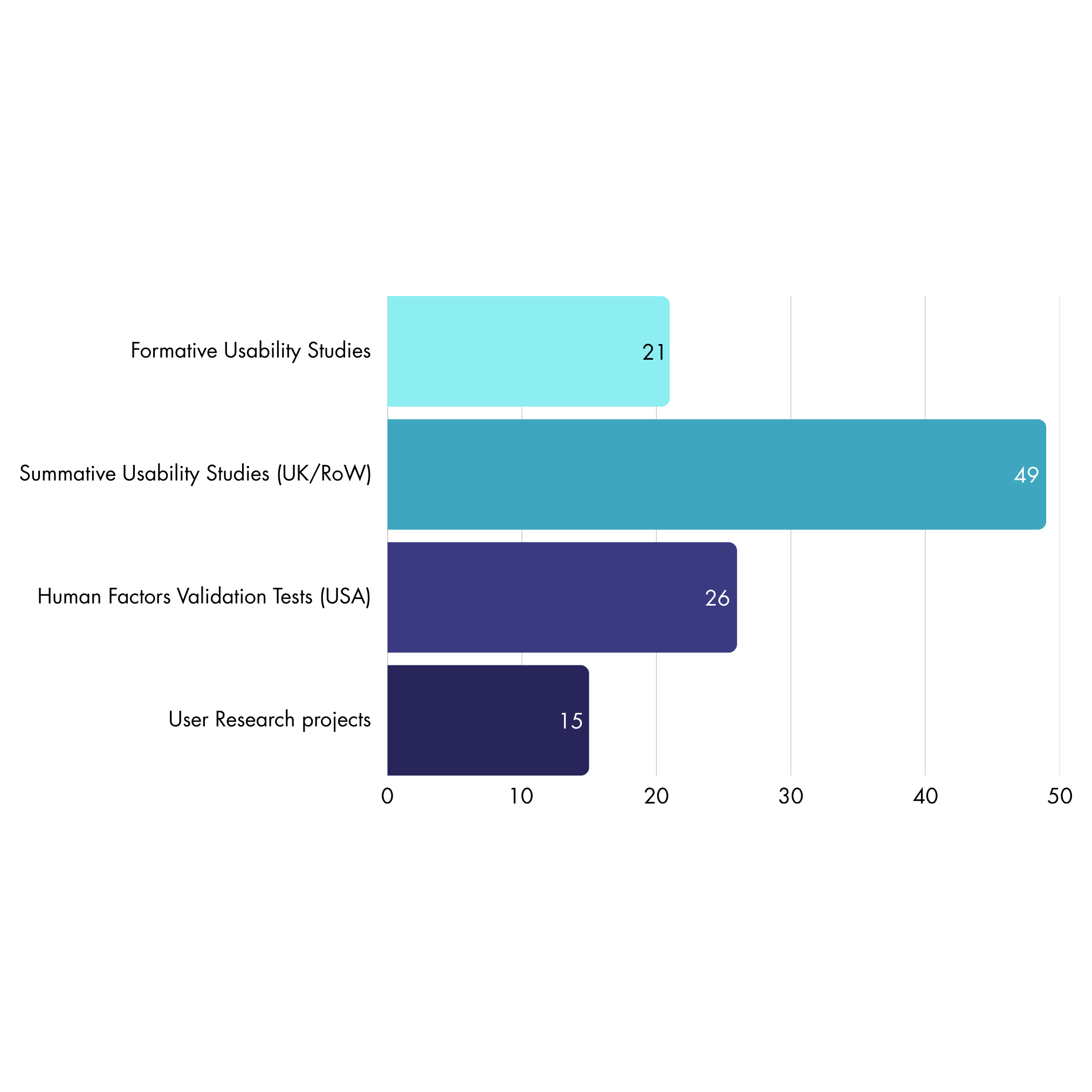Our solutions and services

PROJECT HISTORY
✓ Completion of over 160 projects.
✓ Projects performed in over 10 countries.
✓ Customers range from world's largest MedTech organisations to start-ups.
✓ Every project successful!
OUR CUSTOMERS
75+ global clients
55% of clients return to use THAY Medical.
100% are in healthcare.
We have conducted:
21 Formative Usability Studies
49 Summative Usability Studies (UK/RoW)
26 Human Factors Validation Tests (USA)
12 MDR / Usability Assessments (EU)
15 User Research projects

Total Compliance™ solutions
MDR or IVDR Compliance (IEC62366-1 Annex C)
Readability Studies
Human Factors Validation Tests

Individual Usability™ Solutions
Formative or Investigational Usability Studies
Actual Use and Summative Usability Studies

Human Factors Intelligence™ Solutions
Medical Market Research
Ethnographic Research

User Centric Design Solutions
PIL and Instructions for Use Design, Development & Testing
Packaging Design, Development & Testing
Digital/Software Interface (App) Design, Development & Testing
COMPLIANCES PERFORMED TO:

OUR RECENT PROJECTS
A Summative Usability Study on a Pre-filled Syringe (PFS) system
THAY Medical performed a Summative Usability Study on a pre-filled syringe system in London, UK for a long-standing European customer to aid in their development and subsequent CE Marking submission. The study was performed as planned, to budget and timescales and feedback from the customer stated:
(Q2, 2025)
“Good collaboration, that helped improving the informational materials effectively - 4/5!”
A Safety Guide Study on a In Vitro Diagnostic system
THAY Medical performed a Safety Guide Study on an In Vitro Diagnostic system in the, UK for a long-standing US/UK customer to aid in their development and risk management. The study was performed as planned, to budget and timescales and feedback from the customer stated:
(Q1, 2025)
“We are very happy with the work THAY Medical did on this project. The team are very professional and experienced which helped us overcome challenges that the project faced. We would be very happy to work with THAY Medical on future projects! - 4/5!”